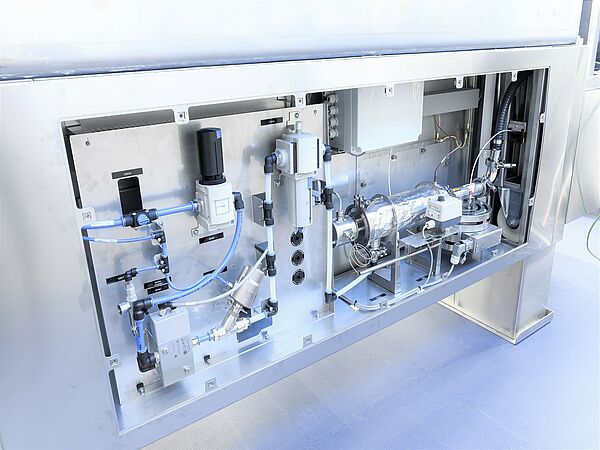
Sterile test isolators from Ortner ensure safe working conditions according to GMP Class A.
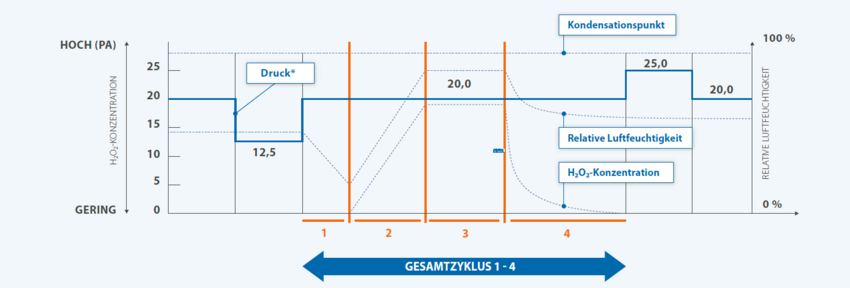
The integrated, fully automatic H2O2 decontamination around LOG6 germ reduction guarantees aseptic working conditions in the isolator.
Safe decontamination of the H2O2 before the start of the test ensures that sterility testing is carried out with the exclusion of false- negative results.

![[Translate to Englisch:] [Translate to Englisch:]](/fileadmin/_processed_/3/c/csm_DSC00524_791b2b8d6d.jpg)
![[Translate to Englisch:] [Translate to Englisch:]](/fileadmin/_processed_/6/9/csm_Einflussfaktoren_CO2_Fu%C3%9Fabdruck_3724c2eaf6.png)
![[Translate to Englisch:] [Translate to Englisch:]](/fileadmin/_processed_/5/b/csm_scientists-working-in-laboratory-2023-11-27-05-15-50-utc_bf2e0e9300.jpg)
![[Translate to Englisch:] [Translate to Englisch:]](/fileadmin/_processed_/b/0/csm__E6A2843_3729d07404.jpg)
![[Translate to Englisch:] [Translate to Englisch:]](/fileadmin/_processed_/6/4/csm_ortner-infineon-Infineon-Chipfabnew-Villach-inside-0921-7_97276e8d57.jpg)
![[Translate to Englisch:] [Translate to Englisch:] Nebeneinander sitzen drei Personen mit Schutzkleidung. Die vorderste Person schaut durch ein Mikroskop. Über das Bild gelegt sind verschiedene Icons, die so aussehen, als würden sie rund um das Mikroskop schwirren.](/fileadmin/_processed_/9/d/csm_adobestock_327970556_web_db394ed81e.jpg)
![[Translate to Englisch:] [Translate to Englisch:] Stefanie Rud und Josef Ortner](/fileadmin/_processed_/8/5/csm_Stefanie_Rud_und_Josef_Ortner_ec6c1b0ec7.jpg)

![[Translate to Englisch:] [Translate to Englisch:]](/fileadmin/_processed_/5/4/csm_Bild2_da3086f97d.jpg)




















 Print out
Print out